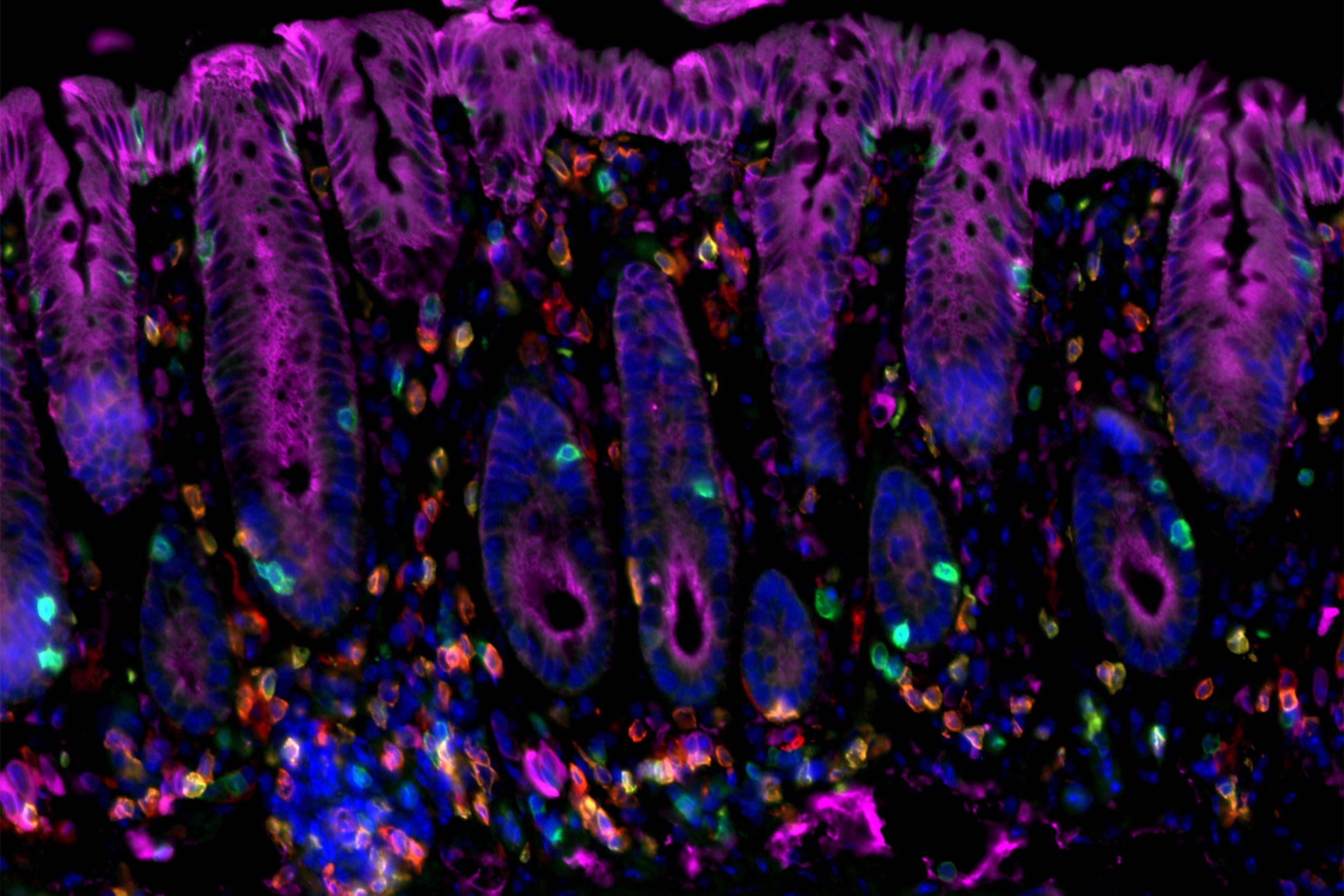
Researchers have identified immune factors that interact to initiate cancer-promoting chronic inflammation — as in this image of cancer-prone colitis in a mouse colon.
Amir Ameri/MGH Center for Cancer Immunology/Cutaneous Biology Research Center
Interaction between immune factors can trigger cancer
Blocking it prevents development of skin and colon cancers in mice with chronic inflammation
A Massachusetts General Hospital (MGH) research team has identified interaction between two elements of the immune system as critical for the transformation of a protective immune response into chronic, cancer-promoting inflammation.
In their report published in PNAS, the investigators demonstrate that elevated levels of the immune factor IL-33 and regulatory T cells (Tregs), which suppress the action of tumor-fighting immune cells, set the stage for the development of skin cancer associated with chronic dermatitis and colorectal cancer in patients with colitis.
“Our research has revealed a critical immunological axis that initiates the development of cancer-promoting chronic inflammation,” said Shawn Demehri of the MGH Center for Cancer Immunology and the Cutaneous Biology Research Center, senior author of the report. “This axis is chronic inflammation’s Achilles heel, and blocking it promises to prevent cancer development in chronic inflammation, which accounts for almost 20 percent of all human cancer deaths worldwide.”
Types of cancer associated with chronic inflammation include inflammatory bowel disease-associated colorectal cancer, hepatitis-associated liver cancer, gastritis-associated stomach cancer, and skin cancers associated with several inflammatory diseases of the skin. The authors note that the activity of certain immune cells — including Tregs, type 2 T helper cells, and macrophages — distinguishes cancer-inducing, chronic inflammation from acute inflammation, which is characterized by the actions of killer T cells and natural killer cells, which protect against cancer.
In their search for factors that may contribute to the transformation from acute to chronic inflammation, the researchers regularly applied an irritating substance to the skin of mice. They observed an increase in the expression of IL-33 — a factor known to alert the immune system to tissue damage and to have a role in allergic reactions — immediately preceding the transition from acute to chronic dermatitis. The presence of IL-33 was found to be required for this transition, and blocking the expression of IL-33’s receptor molecule on Treg cells was found to prevent development of skin cancer in animals with chronic dermatitis.
Increases in both IL-33 and Treg cells were observed in skin samples from patients with chronic inflammatory skin diseases and from patients with inflammation-associated skin cancers. Expression of the IL-33 receptor was also found to be required for the development of colitis-induced colorectal cancer in mice; and both IL-33 and Tregs were found to be increased in colon tissue from both patients with colitis and patients with colorectal cancer.
“We now know that this IL-33/Treg axis is an initiating event in the development of cancer-prone inflammation and that the inhibition of that interaction can prevent inflammation-associated cancer in mice,” said Demehri, an assistant professor of dermatology at Harvard Medical School. “Now we need to determine the efficacy of IL-33/Treg blockade for preventing cancer in patients with chronic inflammation and to test the role of that blockade in cancer treatment more broadly. We’re hopeful that our findings will help reduce the risk of cancer for patients with chronic inflammatory diseases worldwide.”
Amir Ameri of the MGH Center for Cancer Immunology and Cutaneous Biology Research Center (CBRC) is the lead author of the PNAS paper. Additional co-authors are Sara Moradi Tuchayi, Anniek Zaalberg, Jongho Park, Kenneth Ngo, Tiancheng Li, and Elena Lopez, MGH Center for Cancer Immunology and CBRC; Mari Mino-Kenudson, MGH pathology; Marco Colonna, Washington University School of Medicine, and Richard T. Lee, Harvard University.
Support for the study includes National Institutes of Health grants K08 AR068619 and DP5 OD021353 and grants from the Burroughs Wellcome Fund, the Sidney Kimmel Foundation, the Cancer Research Institute, and the Howard Hughes Medical Research Fellows Program.




